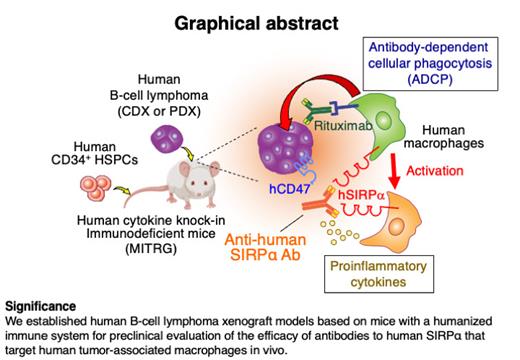
Tumor-associated macrophages (TAMs) are abundant in the tumor microenvironment and are considered potential targets to enhance cancer immunotherapy against B-cell lymphomas. However, faithful and predictive preclinical tumor models of human B-cell lymphomas for the evaluation of therapeutics targeting human TAMs have not been established yet. To examine the antitumor effects of agents targeting human TAMs in vivo, we here established preclinical tumor xenograft models based on Rag2-/-Il2rg-/- immunodeficient mice that express multiple human cytokines, such as M-CSF, IL-3, GM-CSF, and THPO (MITRG mice), and have been reconstituted with a human immune system (HIS) by transplantation of human CD34+ hematopoietic stem and progenitor cells (HIS-MITRG mice). HIS-MITRG mice supported the growth of both the human cell line (Raji)-derived and diffuse large B cell lymphoma (DLBCL) patient-derived xenograft tumors as well as the infiltration of human macrophages into these tumors. We examined the potential antitumor effect of an antibody to human SIRPα (SE12C3) that inhibits the interaction of CD47 on tumor cells with SIRPα on human macrophages and thereby promotes Fcγ receptor-mediated phagocytosis of tumor cells by human macrophages. Combined treatment with rituximab and SE12C3 inhibited Raji tumor growth in HIS-MITRG mice to a markedly greater extent than did rituximab monotherapy in a therapeutic model started after the engraftment of the tumor. This enhanced antitumor effect was dependent on human macrophages and attributable to enhanced rituximab-dependent phagocytosis of lymphoma cells by human macrophages. Treatment with rituximab and SE12C3 also induced reprogramming of human TAMs towards a proinflammatory phenotype. Furthermore, the combination treatment essentially prevented the growth of DLBCL patient-derived xenograft tumors in HIS-MITRG mice. Our findings thus support the utility of HIS-MITRG mice as a model for the preclinical in vivo evaluation of potential therapeutics, such as antibodies to human SIRPα, that target human TAMs.
Disclosures
Saito:Bristol Myers Squibb: Research Funding. Yakushijin:Astrazeneca: Honoraria; Chugai Pharmaceutical: Research Funding; Otsuka Pharmaceutical: Honoraria; Asahi Kasei Pharma: Honoraria; Jazz Pharmaceuticals: Honoraria; Nippon Shinyaku: Honoraria; Janssen Pharmaceutical: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Matsuoka:Nippon Shinyaku: Honoraria; Pfizer: Honoraria; Otsuka Pharmaceutical: Honoraria. Minami:Bristol Myers Squibb: Research Funding; Asahi Kasei Pharma: Research Funding; Otsuka Pharmaceutical: Research Funding; Nippon Shinyaku: Research Funding; Ono Pharmaceutical: Honoraria; Novartis Pharma: Research Funding; Chugai Pharmaceutical: Research Funding. Matozaki:JCR Pharma: Honoraria; Daiichi Sankyo: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal